Notifier un produit cosmétique sur le portail CPNP
La notification électronique d’un produit cosmétique est une obligation imposée par le Règlement cosmétique européen 1223/2009 (article 13). Elle est réalisée avant la mise sur le marché sur le portail informatique de la Commission européenne, le CPNP. Si cette notification ne présente pas de difficultés particulières, il convient de bien la préparer en amont.

Qu'est-ce que le CPNP ?
Le Cosmetic Product Notification Portal (CPNP) est le système de déclaration en ligne de la mise sur marché d’un produit cosmétique. Il centralise ainsi tous les produits cosmétiques présents sur le marché européen, et constitue une base de données interne à la Commission européenne, non accessible au public. En revanche, le CPNP est accessible aux autorités de contrôle des pays membres (à des fins de surveillance du marché) et aux centres anti-poison ou assimilés (à des fins de traitement médical).
La notification d’un produit cosmétique sur le CPNP est un prérequis réglementaire avant la mise sur le marché d’un produit, mais cela n’est pas une autorisation de mise sur le marché. Les informations soumises ne sont pas validées par la Commission européenne, et la notification correcte ne signifie pas que le produit est conforme au Règlement cosmétique. Dès la notification terminée, les données sont mises à la disposition, sans délai, des autorités de contrôle des pays membres et des centres anti-poison ou assimilés. Il n’y a donc pas besoin de notifier au niveau national, sauf dans des cas particuliers (par exemple la déclaration d’établissement en France).
Il est conseillé de notifier un produit peu avant sa mise sur le marché, mais sans s’y prendre au dernier moment.
Les démarches en amont de la notification
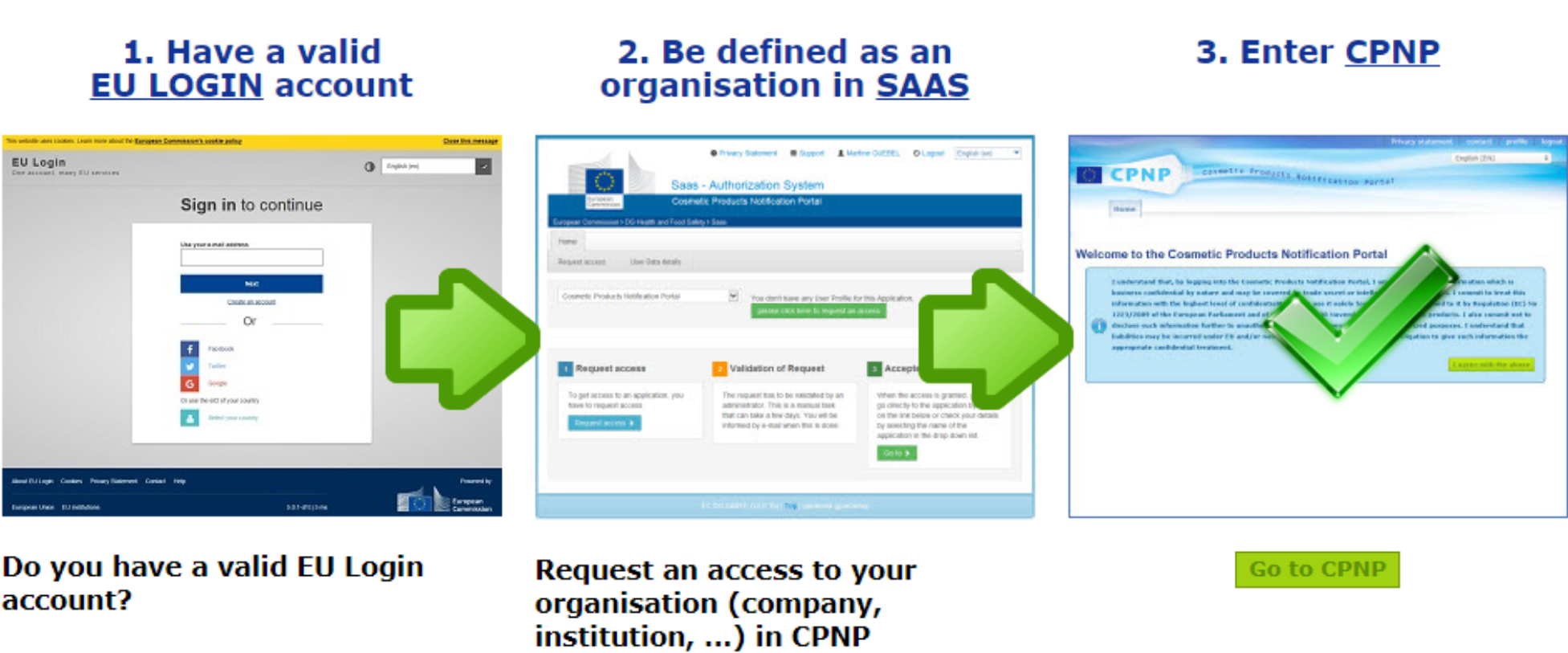
Le Règlement cosmétique prévoit 2 types de notificateurs : la Personne Responsable ou les distributeurs. Avant de se lancer dans la notification sur le CPNP, il est nécessaire de déterminer qui réalise la notification. En effet, dans l’un ou l’autre cas, il est possible de déléguer la notification (par exemple à un prestataire). Mais avant cela, il est nécessaire de demander un accès au CPNP, ce qui comporte plusieurs étapes si c’est la première fois et doit donc être anticipé.
Lien tuto : https://webgate.ec.europa.eu/cpnp/public/tutorial.cfm
La première étape consiste à créer son compte de connexion sur le site de la Commission européenne via l’interface ECAS (EU login account). Une fois le compte créé, un accès au CPNP doit être demandé et créé via l’interface SAAS. C’est l’occasion de créer le compte de la société s’il n’existe pas, qui comportera la liste des produits cosmétiques notifiés et en cours de notification. Il est également possible de rejoindre un compte déjà existant grâce à une liste. Même si la notification est déléguée, la société qui met les produits sur le marché doit donc créer un compte sur le CPNP, et gérer les accès à ce compte. Les identifiants étant personnels, attention à ne pas se retrouver coincé si la personne qui administre le compte quitte la société sans les laisser ! Une fois l’accès au CPNP validé, la notification peut commencer.
Créer son compte : https://webgate.ec.europa.eu/cpnp/public/ecas-create.cfm
CPNP Accès : https://webgate.ec.europa.eu/cpnp/public/saas-start.cfm
Les étapes à suivre pour accéder au CPNP lors de votre première connexion.
Le cas des nanomatériaux :
Lorsque le produit cosmétique contient des nanomatériaux, il doit être notifié sur le portail CPNP au moins 6 mois avant sa mise sur le marché. Cette disposition concerne les nanomatériaux non-inscrits dans les annexes IV (colorants), V (conservateurs) ou VI (filtres UV). Les informations suivantes doivent être transmises :
– L’identification du nanomatériau, y compris son nom chimique IUPAC ;
– Les spécifications du nanomatériau ;
– Une estimation de la quantité de ce nano mis sur le marché chaque année dans les produits cosmétiques ;
– Le profil toxicologique du nanomatériau ;
– Les données de sécurité relative au nanomatériau ;
Les conditions d’exposition raisonnablement prévisibles au nanomatériau.
Ce délai de 6 mois permet à la Commission européenne de demander un avis au CSSC sur la sécurité du nanomatériau en cas de doute.
Comment notifier sur le CPNP ?
La notification du produit cosmétique par la Personne Responsable (ou son délégué) requiert les informations suivantes :
- La catégorie du produit (cohérente avec sa fonction primaire) ;
- Le nom du produit : il doit être suffisamment précis pour permettre aux autorités de contrôle ou aux centres anti-poison de le retrouver spécifiquement. Il doit prendre la forme Marque / Ligne ou gamme / nom spécifique incluant sa fonction. Si ce nom est traduit pour la mise sur le marché dans un ou plusieurs autres états membres, ces traductions doivent être ajoutées ;
- Le nom et l’adresse de la Personne Responsable ;
- Les coordonnées d’au moins une personne physique à contacter en cas de nécessité ;
- Le pays d’origine si le produit est importé ;
- Les substances CMR (1A et 1B) ;
- Les nanomatériaux : identification et exposition raisonnablement prévisible ;
- La formule (encadré) ;
- L’étiquetage original (par exemple le BAT) et une photographie de l’emballage original.
La notification en cours peut être enregistrée comme brouillon et complétée ultérieurement.
Comment déclarer la formule du produit cosmétique ?
La formule du produit à notifier peut être soumise sur le portail sous plusieurs formes :
– La formulation-cadre : il en existe environ 140. Selon la catégorie de produit précédemment sélectionnées, le portail propose une liste. Attention, la notification sous forme de formulation-cadre n’est possible que si la formule correspond aux plages de concentrations mentionnées. Des informations supplémentaires sur certains ingrédients, comme l’éthanol ou les huiles essentielles, peuvent être demandés selon la catégorie du produit ;
– Les concentrations exactes ;
– Les fourchettes de concentrations. Là encore, des informations supplémentaires peuvent être demandées.
Il arrive qu’un distributeur doive réaliser la notification d’un produit cosmétique, lorsqu’il le met à disposition dans un autre état membre et qu’il traduit un ou plusieurs éléments de l’étiquetage de sa propre initiative. Il doit alors soumettre sur le portail CPNP :
- Le nom du produit dans l’état membre d’origine ;
- Le nom du produit dans l’état membre où il est mis à disposition ;
- L’état membre concerné ;
- Le nom et l’adresse du distributeur ;
- Le nom et l’adresse de la Personne Responsable.
Rappelons que la notification doit être enregistrée avant la mise sur le marché du produit. Elle peut être modifiée ultérieurement, car des mises à jour peuvent être nécessaires.
La notification, et après ?
Le produit est notifié, mis sur le marché, mais ce n’est pas la fin des manipulations sur le CPNP ! En effet, la notification doit être actualisée dans certains circonstances. C’est par exemple le cas lorsque la formule ne correspond plus à la formule-cadre initiale ou si la quantité d’un ingrédient est modifiée de façon significative, change de plage de concentration, si un ingrédient à risque est ajouté ou supprimé de la formule.
En dehors des modifications de formule, la notification est mise à jour en cas de :
- Changement de nom du produit ;
- Mise sur le marché dans un nouvel état membre ;
- Nouvelle langue de l’étiquetage ou nom complémentaire ;
- Changement de Personne Responsable ou de ses coordonnées.
Attention également à tenir à jour les coordonnées de la personne de contact. Lorsque le produit n’est plus fabriqué, une case spécifique à cocher permet de prévenir les utilisateurs du CPNP.
Conclusion
La notification d’un produit cosmétique sur le portail électronique CPNP n’est pas la démarche la plus complexe du développement produit, mais elle nécessite néanmoins d’être préparée. Lors des premières, il ne faut pas hésiter à s’y prendre à l’avance, et préparer des brouillons. L’utilisation d’un logiciel de formulation cosmétique peut faciliter la gestion de la formule et des ingrédients spécifiques.
Plus ressources qui pourraient vous plaire





